
Barnes Jewish West County Hospital is a 108-bed facility based in St Louis, MO and is affiliated with Washington University in St. Louis. The hospital's outpatient endoscopy unit performs approximately 6,500 colonoscopy per year and is a referral center for high BMI patients.
Three staff members had undergone surgery due to work-related MSDs. Additionally, 60% of staff reported pain or injury attributed to manual pressure and repositioning.
ColoWraps were used for 271 patients. 252 (92.3%) patients were indicated for ColoWrap use by meeting BMI criteria (> 35); 19 (6.7%) patients were indicated due to prior difficult colonoscopy, abdominal hernia, or multiple prior surgeries. Colonoscopy was completed in 266 (98.2%) patients.
Overall, ColoWrap use resulted in a 73.6% reduction in the use of manual abdominal pressure (50% vs 13.3% of procedures) and a 59.5% reduction (20% vs 8.0% of procedures) in the use of patient repositioning. 100% of endoscopy staff reported significant to very significant reductions in musculoskeletal pain, general fatigue, cecal intubation time, and frequency of prolonged procedures.
All staff also reported a significant to very significant improvement in their ability to focus on other procedural duties during colonoscopy.



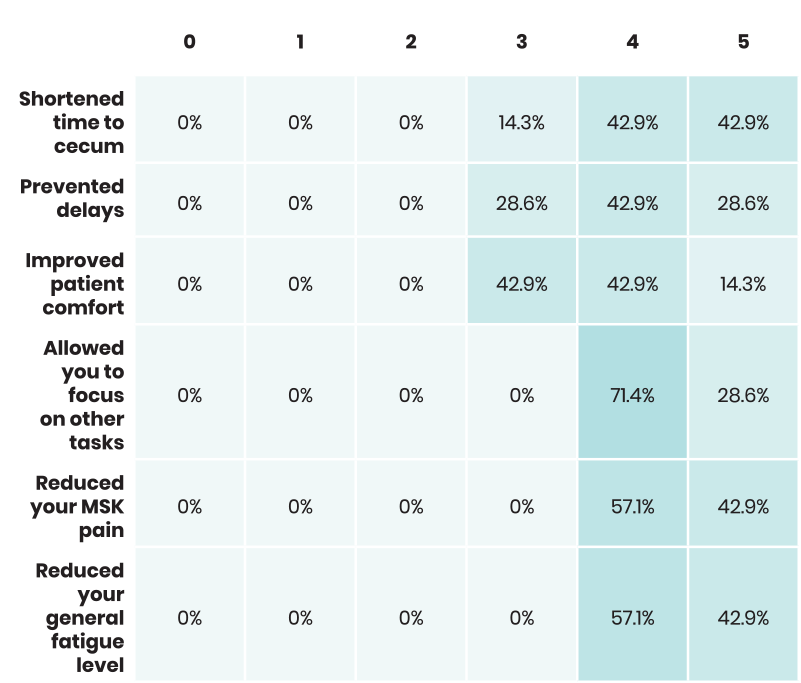
Staff was asked to rank from zero to five, with zero being "not at all" and five being "very significantly", the extent to which ColoWrap performed. Below are those results.
This was a single center observational study. In September 2020, a baseline assessment found that manual pressure and patient repositioning were being used in 50% and 20% of colonoscopies, respectively. Three staff members had undergone surgery due to work-related MSDs and 60% of endoscopy staff reported pain or injury that they attributed to manual pressure and repositioning. BJC West County adopted the following patient criteria for ColoWrap use: BMI > 35; prior difficult/incomplete colonoscopy; abdominal hernia; or multiple prior abdominal surgeries. Procedure data, including colonoscopy completion, procedure time, and frequency of manual pressure and repositioning were tracked for each case in which a ColoWrap was used through 05/2021. Staff completed a satisfaction survey in 06/2021. We aimed to determine if use of the ColoWraps would reduce the need for manual pressure and repositioning and positively impact staff safety and satisfaction.
In this observational study, use of the ColoWrap Colonoscopy Compression Device in patients with BMI > 35 history of difficult colonoscopy, abdominal hernia, and/or multiple prior abdominal surgeries reduced the need for manual abdominal pressure and patient repositioning as compared to a baseline assessment. Endoscopy staff reported reductions in musculoskeletal pain, fatigue, and frequency of prolonged procedures with ColoWrap use.
References
1. Biggers L. Endoscopy Staff Injury: A Serious Problem Hidden in Plain Sight. International Journal of Safe Patient Handling & Mobility. 2018;8(4) 2. Crockett S, Dellon ES, Biggers L, Ernst DA. Use of Patient Abdominal Compression Device Reduces Staff Musculoskeletal Pain Associated With Supporting Colonoscopy: Results From a Randomized Controlled Trial. Gastroenterol Nurs. 2021 Mar-Apr 01 2021;44(2):136-145. doi:10.1097/SGA.0000000000000550
7310 Millhouse Rd.
Suite #175
Chapel Hill, NC 27516
Call us: 1-888-815-3376
Copyright © 2025 ColoWrap, Inc. All Rights Reserved.